When designing mechanical systems for nonsterile solid dosage drugs, there is more to consider than just the design/selection of the actual HVAC equipment. There are many factors and components of the system design that need to be considered for those systems. While many design requirements associated with current good manufacturing practice (cGMP) spaces are required by the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and others, the engineer must be aware of several factors when designing the mechanical systems for these facilities.
Pressure adjacencies
Pressure adjacencies are critical in the design of manufacturing suites, packaging spaces, and other cGMP areas. The design strategy for pressure adjacencies is not a one-size-fits-all subject and should be based on the parameters and characteristics of the specific application. Too often, the design engineer will default to a design strategy that starts with the most critical area being the most negative. That is not always the best direction, especially when the process does not contain hazardous material but is dust-generating. In most areas where solid dosage drugs are manufactured, not only does the product need to be contained, but the space must be controlled as a "clean" environment.
There are many strategies for pressure cascades in cGMP spaces for nonsterile environments. While the "bubble" airlock is an effective strategy for this type of application, a risk analysis should be performed for each specific project to determine the best approach.
The three common types of pressure adjacencies are bubble, cascade, and sink.
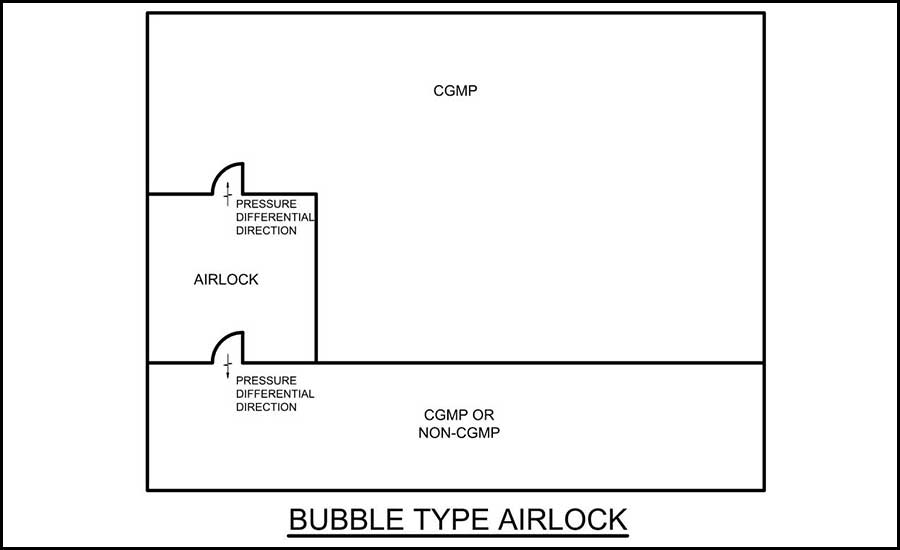
Bubble airlock — For bubble airlock design, the airlock is positive to the adjacent spaces on each side of the airlock. While the cGMP space is negative to the airlock, it should still be positive to atmospheric or other adjacent spaces. See Figure 1 for an example of this layout.
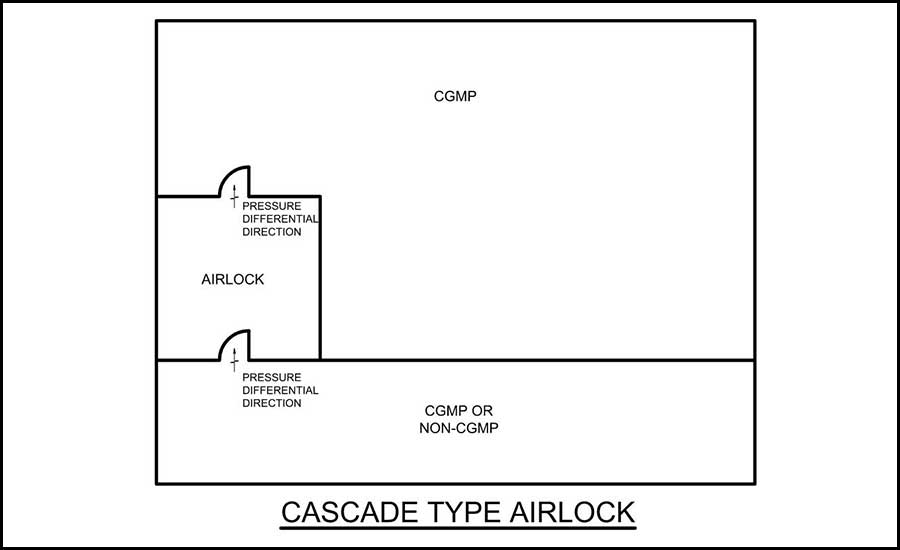
- Cascade airlock — For this layout, the area of pressure continues to become more negative as you proceed from one side of the airlock to the other. This type of layout is more common for hazardous processes (see Figure 2).
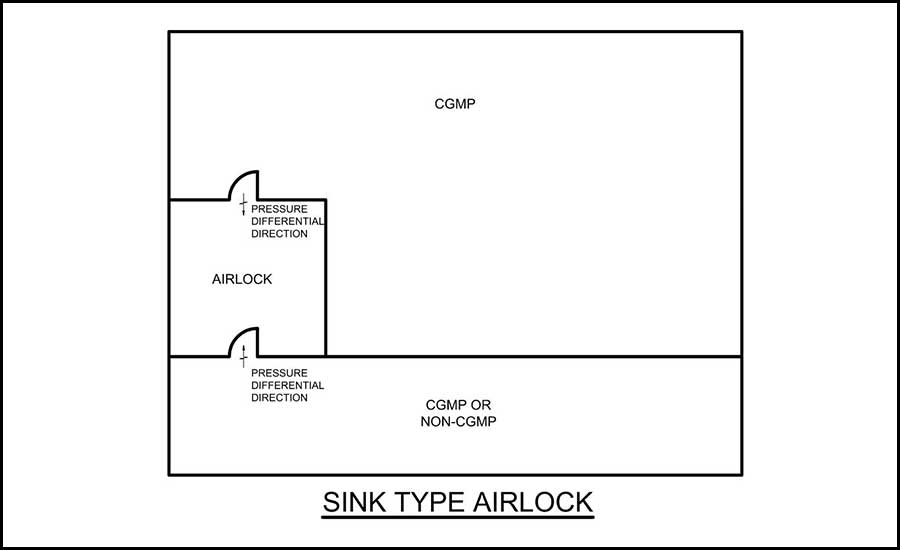
- Sink airlock — For this layout, the airlock is the most negative of the three spaces, where each space adjacent is positive to that airlock (see Figure 3).
The following are some typical nonsterile areas and design considerations for the pressure adjacencies:
- Areas with open/exposed product (cGMP) — The suite with open product should have an airlock/ante room for entering that suite even if coming from other cGMP areas. That airlock can also be used for gowning. From a pressure cascade perspective, the airlock would be the most positive with the suite and the adjacent space (cGMP — unexposed product) being less positive. The suite should also be positive to atmospheric pressure. This type of cascade is commonly called the bubble cascade.
- Areas with unexposed product (cGMP) — The suite with product that is not exposed (but still cGMP) should have an airlock (gowning) to the non-cGMP areas. The pressure cascade would be similar to exposed product suites. The airlock would be the most positive space with the non-cGMP space and cGMP space as less positive than the airlock. The cGMP space should also be positive to atmospheric. As mentioned above, this is the bubble cascade.
Cross-contamination is always a concern with manufacturing plants that have multiple product streams. Typically, each product stream cannot be exposed to the next one. The same pressure cascade approach would apply, but to help with the cross-contamination risk, the spaces shall be installed as airtight as possible. The following are some space design considerations when developing pressure cascades:
- Seamless floors.
- Gasketed doors and ceiling plans (if installed).
- Potential leaks — sealing around all penetrations leaving or entering the suites, including door frames and receptacles.
- Some wall types will have a greater porosity for leakage.
- In airlocks, the doors should be interlocked so only one door can be opened at a time.
A note on pressure differentials: In general, for nonsterile cGMP spaces, a pressure differential of 0.03 inches of water column (wc) is an acceptable minimum. As pressures will always fluctuate some, the design engineer should target a higher differential pressure than the allowable minimum. By designing for 0.04 wc to 0.06 wc, one allows for a safety margin. For more critical spaces as determined by a risk analysis, the pressures may need to be even higher.
Humidity
Humidity control is a must for some solid dosage products being manufactured. Since the spaces are typically validated and humidity is checked regularly, the approach most engineers take is to design the space for at least 5% relative humidity (RH) lower than the allowed maximum. This allows for fluctuations in the space due to infiltration, seasonal swings, manufacturing processes, and other factors.
Many facilities in the pharmaceutical industry struggle to maintain humidity levels when tight control is a requirement of the process/product. These may be due to the following:
- No vapor barriers in the walls, ceilings, etc. Even if the adjacent spaces are interior, the walls will need vapor barriers to help with the control of humidity, unless the adjacent space is maintained at the same humidity level.
- Building management system (BMS) controls — Many of these spaces have a high level of air changeover because of heat gain or needed air quality. Sometimes the HVAC systems will end up “hunting” regarding humidity controls. The air handler is constantly increasing/decreasing discharge air temperatures and the heating/reheat follows along with it. As these types of spaces typically have a high air change rate, the discharge temperature into the space is only a few degrees below the space temperature set point. To verify constant and continual control of humidity, the air handler should still dehumidify the airstream and then apply reheat for the temperature trim. Also, desiccant technologies are available to help with humidity swings and control. These types of systems should be considered when budgets allow.
- Infiltration from operators passing through the space can also affect the humidity in validated controlled spaces. To help alleviate those issues, an airlock that is controlled to the same humidity levels can be useful. If there is no room to add an airlock, controlling humidity in that adjacent space where the operators enter would dramatically help.
- If a humidifier is needed for the space to maintain minimum humidity requirements, design considerations for that system are needed. It is beyond the scope of this article to discuss how to design such a system, but for cGMP spaces, mold is always a concern. Care needs to be taken when these systems are designed, so excess moisture is not found in the ductwork, mechanical spaces, or the actual cGMP spaces. Typically, the cGMP spaces are regularly tested for mold, and if moisture is present over a long period (or sometimes short), mold could be found.
As with temperature control discussed above, these spaces that are humidity controlled should have their data recorded and stored in a validated system. Also, as with the thermostats, the space humidistats need to be added to the preventative maintenance schedule, as these devices need to be calibrated periodically.
Temperature
Many solid dosage drugs require specific temperature ranges for the product to be manufactured properly and packaged or stored in the warehouse. Some do allow excursions within a given range. With most of these types of spaces needing to meet cGMP standards, the spaces typically go through validation and monitoring throughout the manufacturing, packaging, and storage processes.
Temperature control is important. If the environment drifts out of the specified range, there could be waste in the manufacturing process, i.e., disposal of product, recalls, and other issues. During the design of these spaces, temperature control should be reviewed very closely to achieve the optimal temperature range.
For the design to successfully pass validation, during the design process, the entire space needs to meet the required temperature range through a year’s worth of seasons. The design should incorporate an evenly widespread supply air distribution, as all areas of the space will need to remain within the required temperature range (from floor to ceiling). Close review of the local heat gains will need to be reviewed and calculated, so supply/return air systems can quickly dissipate those warm spots with only minimal impact to the space temperature.
Many spaces don’t pass validation because the design does not take into account the fact that similar temperatures are required throughout the space — from floor to ceiling. The design needs to ensure there are no dead spaces from an airflow/temperature perspective. It is common for the design engineer to target only the large equipment heat load in the space as opposed to the entire space.
A preferred design method for these critical spaces is to supply air with laminar flow from above and place the return air grilles at floor level. This design will also help with particle counts. Other designs can also be incorporated, such as supplying and returning from the ceiling, but these methods usually require a higher air change rate.
Once the space is designed for temperature control, the BMS should monitor and record the temperatures. The BMS should be validated so the data can be utilized for the manufacturing process. Systems that are not validated should have a separate validated environmental monitoring system (EMS) where the data can be recorded and stored.
Once the temperature sensors are installed in the space, they need to be added to the preventative maintenance schedule, as these devices need to be calibrated periodically. Without calibration, the product in the plant may not be in an environment that is meeting the needed temperature range.
Air changes per hour
Many cGMP production areas are designed with higher air changes per hour (ACH) than needed. While most pharmaceuticals have minimum requirements of six ACH, many spaces can be designed for less and still provide an effective ACH. This helps with energy savings for the plant.
Rates higher than the minimums are usually only needed when there is a larger heat gain that needs to be dissipated, or when the manufacturing process needs dictate less air particles in the space, i.e., cleanrooms and other critical spaces. Those spaces typically need higher ACH rates to meet ISO requirements. Another scenario where this applies is when there is a large amount of dust collection in the space that will require makeup air. Even though there may be a few spaces in the plant that need the elevated cleanroom requirements, most cGMP spaces should be designed at the four to six ACH range in the absence of other specific requirements. Targeting lower air change rates will allow for a much “greener” design.
Filtration
Filtration can play an important role in the success of many cGMP areas for nonsterile manufacturing. It is also a great tool to reduce cross-contamination.
When a single air handler serves two product streams and the unit has return air, HEPA filtration can reduce the risk of cross-contamination. Also, each time the HEPA filters are replaced on their regular preventive maintenance schedule, they should be leak tested to verify the filters do not have any holes and they are seated well in the frame. Ideally, to minimize the risk of cross-contamination in critical spaces, separate air handlers are suggested.
In areas where the product is not exposed and there is no risk of cross-contamination, HEPA filtration is not always required. Many plants will use HEPA more frequently than needed. However, with the cost of preventive maintenance of HEPA filters, they should only be utilized where necessary.
There are places in the plant where HEPA filters should be installed in the air-handling system and others where they should not. The discussion below also assumes a nonhazardous product — much of the nonsterile product streams fit this classification, but not all. With every new project there should be a review to verify what is specifically needed for each space.
- Manufacturing/processes with open product — HEPA filtration is suggested to help reduce particles in the air, thus reducing contamination of the manufacturing process. Risk analysis should be performed. Many spaces end up not needing HEPA to achieve the designed cleanliness because of the product requirements.
- Packaging where there is exposed product — HEPA filtration is suggested to help reduce particles in the air, thus reducing contamination of the manufacturing process. Risk analysis should be performed. Many spaces do not need HEPA to achieve the designed cleanliness because of the product requirements.
- Packaging with no product exposure — Perform a risk analysis of the space as HEPA filtration may not be needed as there is no exposed product. Many spaces do not have HEPA filters as the level of desired cleanliness can be achieved without them.
- General cGMP areas, cGMP warehouse, raw material storage (sealed) — Perform a risk analysis of the space as HEPA filtration most likely is not needed if there is no exposed product.
Even if it is determined that HEPA filtration is not needed through risk analysis, at least 95% filters and 30 % pre-filters should be used throughout the plant. This level of filtration would be for spaces with outdoor air and return air, but not return air from different product streams.
Conclusion
There are many HVAC design considerations for the construction and renovation of a new suite in a nonsterile pharmaceutical environment. When starting down the design path, it is always important to involve the users in the design and review the risks for each design decision.