Hospitalization per se is now the third-leading cause of death in the U.S. How did this happen? As analyses of in-patient care and hospital spending moves forward, it has become clear that patients too often become sick from the very act of being a patient. In 1999, the U.S. Institute of Medicine published, “To Err is Human: Building a Safer Health System,” a landmark article that revealed the huge burden of harm to hospitalized patients. This article presented the number of patients injured or killed from errors in hospitals: errors such as wrong site surgery, patient falls, incorrect medication administration, and exposure to microorganisms causing serious infections — infections that were not present before the person donned a johnnie and became a patient. Each year, these medical errors and healthcare-associated infections (HAIs) kill hundreds of thousands of patients and costs healthcare millions of dollars.
The components of this epidemic of harm in hospitals are multifold. More people survive from successful medical intervention to become vulnerable patients, often with impaired immune defenses. The select microorganisms that are not killed by powerful antibiotics used in hospitalized patients survive because of genetic or physical resistance. These resistant organisms subsequently reproduce, thriving in patients, on surfaces, and in hospital mechanical systems. In this way, the hospital unwittingly becomes a reservoir and vector for virulent, treatment resistant pathogens. That is the bad news.
The hospital building manager plays a key role in saving patient lives and hospital dollars. The good news is that the building manager can improve patient healing, save lives, and reduce avoidable hospital expenses.
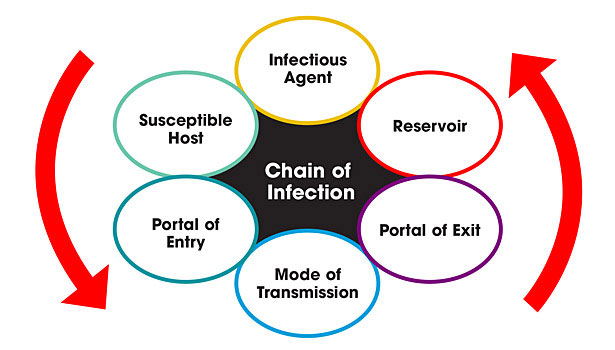
This article will: 1) review the transmission of infectious microorganisms which cause HAIs, with emphasis on the role of indoor air and the built environment in the chain of infection; 2) review current standards and guidelines for indoor air management; and 3) offer guidelines for evaluating and optimizing current and new air cleansing systems marketed to diminish HAIs.
The pathways of infectious disease transmission in HAIs
Throughout medieval and renaissance history, until the mid-nineteenth century, virtually all infectious diseases were thought to be transmitted through the air, leading to names of diseases such as malaria. This concept ended abruptly when the microbial nature of infectious disease was recognized with the invention of the microscope. With the visualization of germs, the role of contact spread in infectious disease transmission rapidly gained acceptance and quickly eclipsed the study and prevention of airborne transmission.
In 1910, Charles Chapin, in his classic treatise “The Sources and Modes of Infection,” wrote, “Without denying the possibility of such airborne infection, it may be fairly affirmed that there is no evidence that it is an appreciable factor in the maintenance of most of our common contagious diseases. We are warranted, then, in discarding it as a working hypothesis and devoting our chief attention to the prevention of contact infection.”
Despite scientific and research evidence that some diseases, such as tuberculosis, were transmitted primarily through the air, Chapin’s governed conventional epidemiologic thinking over the next 40 years. In 1935 William Firth Wells, then an engineer at Harvard University, began to challenge this dogma. He argued that certain diseases, most particularly measles and tuberculosis, were spread through the air by tiny droplet nuclei and thus were truly airborne.
We now know that large quantities of infectious particles are expelled during routine patient bodily functions common in hospitals. In the healthcare setting viruses, bacteria, and fungal spores are generated and released by human speech, coughing, sneezing, and skin shedding.
Transmission of disease from these sources can result from direct contact with an infected person or from indirect contact through an intermediate object. An infection caused by direct contact could stem from caregivers not washing hands prior to attending patients. Another form of direct contact transmission of disease is by droplet transmission, where large expelled droplets settle onto a surface quickly, typically within three feet of the source. In order for an infection to be caused by droplet transmission, a susceptible patient must be close enough to the infected individual for the infectious droplet to make contact fairly quickly.
Many of these pathogens can spread both via the direct contact droplet route and through indirect airborne transmission if droplets desiccate in dry air and become aerosolized. Research has shown that infectious particles in small aerosolized droplets can become airborne and remain suspended in air for long periods of time, dispersing disease over great distances and ultimately causing illness in individuals who have had no contact with the infectious source. Transmission of infectious disease by the airborne route is dependent on the interplay of several critical factors, primarily the diameter of the particle and the extent of desiccation.
Large particles with a diameter greater than 5 micrometers (mm) fall out of the air, and smaller particles remain airborne. Another critical variable is the rate at which particles desiccate. Even large, moisture laden droplet particles desiccate rapidly. In his seminal paper, Wells showed that up to 50 mm can desiccate completely within 0.5 seconds. Rapid desiccation is a concern because the smaller and lighter the infectious particle, the longer it will remain airborne. Even when infectious agents are expelled from the respiratory tract in a matrix of mucus and other secretions creating large, heavy particles, rapid desiccation creates droplet nuclei typically 0.5–12 mm in diameter, lengthening the time they remain airborne. By this drying mechanism, very large aerosol particles may initially fall out of the air only to become airborne again once they have desiccated.
An additional challenge to hospital infection control practitioners is that large-sized droplets can also remain suspended in air for long periods in certain conditions. Droplets settle out of air onto a surface at a velocity dictated by their mass. If the upward velocity of the air in which they circulate exceeds this velocity, they remain airborne. Hence, droplet aerosols up to 100mm diameter have been shown to remain suspended in air for prolonged periods when the velocity of air moving throughout a room exceeds the terminal settling velocity of the particle.
Healthcare facilities in particular face a unique challenge with infection control because of high densities of both contagious and immune-compromised patients in the same building. Many of the airborne microorganisms in hospitals are increasingly found to have developed strong drug resistance paralleling a rise in the quantity and variety HAIs. Not surprisingly, HAIs have become ubiquitous and healthcare facilities are now a common source for highly drug-resistant pathogens.
While recommendations for hospital hygiene include hand, instrument, and surface hygiene for interruption of these vectors, even excellent adherence to these hygiene protocols do nothing to stem the transmission of infectious airborne particles. Even with a list of known pathogens that can cause HAIs (and evidence that many are at least in part transmitted through the airborne route), the extent to which airborne transmission contributes to the overall HAI rate continues to be debated. Microbiologists conclude that at least one-third of all HAIs involve airborne transmission at some point between the origin and the susceptible host, however healthcare facility reports contain varying data about the extent to which airborne transmission contributes to total HAIs.
Review of current standards and guidelines for indoor air management
The complex hospital environment requires special attention to ensure healthy IAQ to protect patients and health care staff against HAIs and occupational diseases. Maintaining a high level of IAQ has become an increasing challenge for health care facilities. At least 7% of all patients who enter an inpatient healthcare facility for treatment will develop an HAI.
Because airborne contaminants are known to cause infections in hospitals, it is imperative for design engineers and building managers not only to control air movement in the space, but also to ensure that the cleanliness of delivered air prevents the growth of microorganisms which cause infections.
Healthcare facilities are subject to numerous regulations and requirements relating to performance of their HVAC systems. Based on these regulations, hospitals currently attempt to reduce airborne infectious disease transmission by: 1) clearing airborne contaminants near their source through local exhaust ventilation; dilute and remove contaminants generated in certain areas such as operating rooms and patient rooms by room air changes (RAC); 2) create directional airflow by establishing a pressure differential between spaces and forcing air to flow from the protected space to the less protected space through positive and negative room pressurization; 3) utilization of cleaning elements such as HEPA filters and ultraviolet light in ventilation systems; and 4) creation of barriers and pressure differentials for construction and demolition zones.
Guidelines for evaluating and optimizing systems marketed to diminish HAIs
Have hospital ventilation guidelines evolved to protect patients from dangerous airborne infectious diseases? What are the goals for HVAC regulations and how are these goals accomplished? With these questions in mind, we can review the current standards and common practices as well as evaluate new technologies marketed to cleanse air and air handling systems.
The following are some guidance on how to determine the validity of new solutions so healthcare money will be wisely spent on effective products. New products are ubiquitous in the HVAC market — ultraviolet light to kill mold, smaller and smaller pore filters to retain microorganisms, and ionization to kill bacteria. There is also a burgeoning area of new surface materials marketed to either impede the growth of organisms or kill them on contact. The efficacy of these solutions should be analyzed to determine the real value of the solution. How do these solutions work together? What data supports the statistics? Often “common sense” and “wishful thinking” are the backbones of the presentation of data. How can we determine if these strategies actually work?
Steps to understand and analyze infection control systems presented by vendors:
- Where have the studies quoted been published? Respected professional publicationshave more stringent standards than marketing brochures.
- Don’t be intimidated by scientific words. A series of four-syllable words does not mean the “solution” delivers as promised. If a vendor is using vocabulary you do not understand, ask them to slow down and explain the system clearly.
- Question the data that is presented to you. Do the test methods support the claims? For example, are changes in skim milk proteins equivalent to reduced patient infections?
- What are the purported results from using the new system? If the solution claims to decrease patient HAIs, look carefully because very few good, controlled studies have been done.
-
What is a good study?
- Retrospective versus prospective: studies looking forward in time are preferable to those looking back.
- Is the control group appropriate for the conclusions?
- Are the results analyzed for statistical significance with p-tests or t-tests?
Conclusion
HVAC maintenance is critical to not only the comfort, but also the health of the hospital occupants — the patients and staff. Think about the long-term evolutionary consequences of infection control strategies. A bacterial or viral organism can reproduce at alarming rates, so under the pressure of antibiotics and antiviral medications the organisms that survive are not only more virulent, but they have less competition for nutrients and can reproduce rapidly. The bottom line is that we need to think carefully about the strategies we employ to kill organisms not only from the perspective of how well they work now, but also from the perspective of what capabilities the survivors will pass on to the progenitors of future cell lines. In part 2 of this article, we will consider some of he characteristics of indoor air, such as relative humidity and temperature, which can be managed to decrease the airborne spread of infections and therefore increase patient safety.