As engineers work to reduce the risk of Legionella growth in buildings, what strategies can be borrowed from treating illnesses caused by biofilms?
Thanks to genetic analysis tools, we can study bacteria in their natural habitat rather than in the artificial environment of a glass petri dish. What have we learned from this new, voyeuristic approach? We now know that bacteria do not live alone, instead forming complex societies with differentiated work tasks and sophisticated mechanisms to protect their communities from antibiotics and environmental stresses.
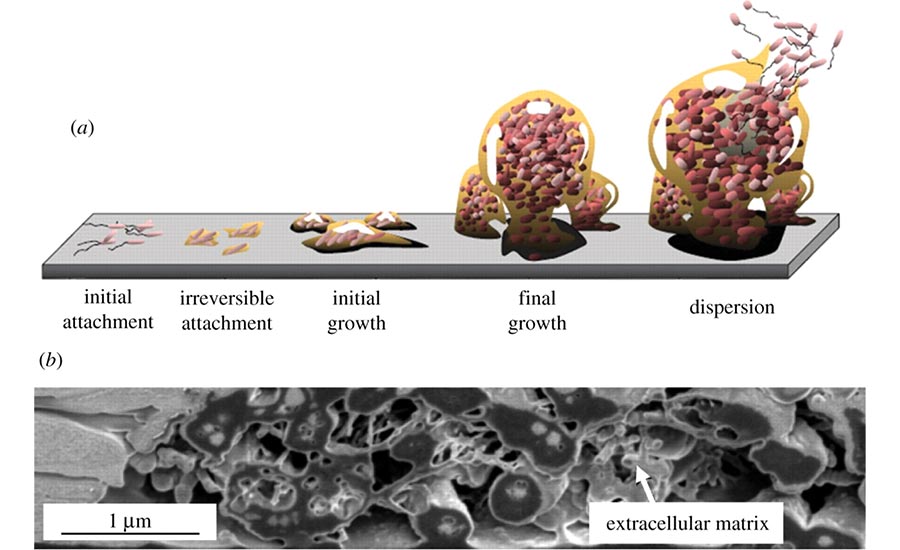
How does this happen? When a roaming single bacterial cell finds an inviting surface, it undergoes dramatic lifestyle changes. Losing its ability to propel itself freely as it settles down in the new environment, the bacterium reproduces rapidly and begins to establish a stable (yet diverse) biofilm community. Once the advance party of bacteria has created this immature biofilm, they selectively invite or reject single roaming cells with chemical signals such as cyanide and natural antibiotics. Soon, a communal population creates sophisticated architecture, including mushroom-like structures blossoming on submerged surfaces or towering projections shooting upward from surfaces exposed to air.
While engineers have long recognized the tenacity of bacterial, fungal, and algae biofilms surviving both inviting and hostile conditions, those of us in the medical sciences are only recently fully appreciating the complexity of managing them.
Biofilms made up of thousands of different microbial species colonize our skin, teeth, and surfaces of our intestines and airways. While most promote human health through digestion and a first line of defense against invading pathogens, not all are beneficial.
When foreign objects in our bodies — such as intravenous catheters or joint prostheses — become covered by these films, they can act as bacterial reservoirs, sending pathogens throughout our bodies and causing dangerous systemic disease. Additionally, the protected biofilm-associated microbes are particularly resistant to many antimicrobial agents, making these infections difficult to treat.
Medical research has developed interesting strategies to control biofilms, for example:
-
Exposure to enzymes that degrade the polymeric foundation of biofilms, which results in the release of components which are easily cleared by immune cells.
-
Conduction of electrical currents in combination with electromagnetic fields and ultrasound, resulting in a substantial reduction of living cells in staphylococcus and pseudomonas biofilms.
-
Surface coatings with metals, antiseptics, or simply very slippery materials effectively eradicate or block bacterial biofilms on the surfaces of endotracheal tubes and catheters.
Working together with new metagenomics tools, clinicians and building engineers can interrupt the ability of harmful bacteria to form biofilms, thereby helping to reduce the spread of dangerous, treatment resistant infections. ES