These systems have been integrated over the decades with improvements in filtration processes, and in recent years with a resurgence of ultraviolet germicidal irradiation (UVGI) applications, to form a triad of technologies used by engineers to provide acceptable IAQ in health care facilities.
Although insightful, Nightingale could not have foreseen all the mounting challenges facing health care facilities in this new millennium. Today's escalating fears, from TB epidemics to bioterrorism with aerosolized anthrax spores, have generated even greater demands on this technology triad to provide a healthy indoor environment. That environment must be conducive both to patients (to nurture well-being during diagnosis treatment, and recovery), and to health care workers (to reduce exposure to patient pathogens and to limit sensitization to allergenic substances).
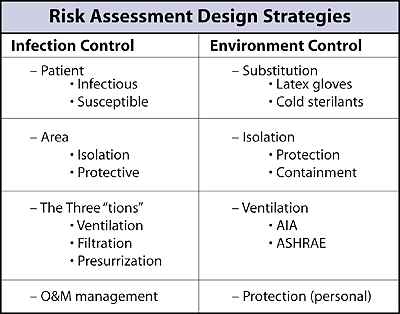
The strategy of choice in health care facility design is one of risk assessment that emphasizes either infection control3 or environmental control4. As shown in Table 1, these two approaches share many common elements but are initiated differently. Infection control begins with the ill patient and the at-risk health care staff, while environmental control starts off with the causative agent of patient illness and health care worker exposure or sensitization.
These risk assessment approaches bring to bear skilled practitioners from the medical and building sciences, including architects, engineers, epidemiologists, and industrial hygienists. These multiple disciplines are ultimately responsible for providing the effective IAQ practices in health care facilities today. Where do these practitioners go for health care facility design criteria?
Three Prevailing Guidelines and Standards
Architects look to the American Institute of Architects (AIA) and its Guidelines for Design and Construction of Hospital and Health Care Facilities5for design criteria. Most state health agencies invoke these AIA Guidelines via the Joint Commission on Accreditation of Health Care Organizations (JCAHO) certification process.The latest edition (2001) of these AIA Guidelines has notable changes to the number of ach for selected rooms in health care facilities, as shown in Table 2, to address both diagnosed infectious patients in isolation rooms6, and, more broadly, undiagnosed persons, such as those with TB, in waiting areas and procedure rooms 7.
Total ach's, consisting of both fresh ventilation air and filtered recirculation air, are identified by specific room type along with any pressurization and exhaust requirements. These guidelines do however lack specificity on hvac equipment design requirements.
So engineers look to the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE) Handbooks and Standards 8,9 to "fill in the blanks" in the AIA Guidelines. Some state health agencies also cite ASHRAE Standard 62 "Ventilation for Acceptable Indoor Air Quality."
But Standard 62 lacks essential detail as well, such as ventilation rates for certain health care applications or procedures. ASHRAE Special Project (SP) 91 Health Care Facilities Design Guide is presently addressing coordination of AIA and ASHRAE guidelines and standards for health care facilities with drafts of a new ASHRAE publication, Hospital HVAC Design Guide, currently undergoing reviews. Note that additional OSHA standards and NIOSH criteria may also apply to employee-only accessed areas of health care facilities.
Epidemiologists and industrial hygienists look to the Centers for Disease Control (CDC) for the latest developing practices for controlling the spread of diseases. These practices appear in the CDC Guideline for Environmental Control in Health Care Facilities 10 and are often a precursor to future AIA Guidelines.
Three Major Risks
The nature of pathogens range from viruses to bacteria to fungi, and their modes of transport vary from airborne to waterborne to surface-borne. Improving IAQ can only directly address those pathogens that are airborne. However, controlling air conditions, such as humidity within a preferred range (30% to 60% rh), can have indirect, mitigating effects on the propagation of surface-borne pathogens as well.Typically, nonporous and untextured floor, wall, or ceiling surfaces are not platforms for pathogenic microbes unless the surface becomes moist, sticky, or damaged. Once dried, cleaned, or repaired, the microbes tend to disappear. Major exceptions to this are floor carpets and suspended ceilings, which can harbor significant populations of microbes and such furnishings must be precluded from use in higher risk patient areas 11.
If properly disinfected, through chemical treatment, thermal eradication, UV irradiation, or metal ionization, any threat of waterborne microbes in health care potable water supplies and water sources such as cooling towers can be dramatically limited11.
That returns us to the airborne side of the risk picture. One of the major airborne risks facing health care facilities is respiratory pathogens. The time-tested method for reducing the rate of airborne infection is dilution with ventilation or fresh air. What Nightingale suspected in the 1850s was quantified a century later in the 1950s by the team of Wells and Riley. The Wells-Riley equation6 quantifies the relationship between higher ventilation rates and lower infection rates as illustrated in Figure 1.
As the graphic illustrates though, the benefits of ventilation air diminish as the relationship asymptotes out, never allowing one to eliminate infection completely, especially within practical limits of ventilation system design, operation, and cost. So other complimentary technologies, filtration and irradiation, are brought to bear to reduce further risk of infection from respiratory pathogens.
The IAQ Technology Triumvirate
So obviously, ventilation alone is not the answer to better IAQ in health care facilities. A sound approach to acceptable IAQ is to use ventilation in conjunction with: air filtration on recirculated and fresh air, using mechanical arrestance media to clean air of microbial (and other particulate) matter; and irradiation in targeted applications, using UV emitters to alter airborne (and surface-borne) microbe DNA and limit the procreation of those infectious agents.Ventilation
Ventilation air is critical to establishing proper pressurization or differential airflows that are essential to protecting patients and health care workers from infection by airborne diseases. As noted in Table 2, isolation rooms with large fresh ach's negative pressurization and 100% exhausted return air are the primary control in health care facilities for containing the spread of airborne disease, like TB, from diagnosed, infected patients.Conversely, immune system-compromised patients must be protected from infection, such as aspergillosis, by oversupplying these rooms with sufficient makeup air to support adequate positive pressurization. Most exhaust air fans for isolation areas are dedicated to this use, but most supply air systems are not. Hence, due to demands elsewhere in the health care facility, ventilation air supply may be subject to variation and pressurization compromised in these rooms12.
Over the course of the last decade, a growing trend has emerged to condition ventilation air with DOAS as well. In the cooling mode, these systems decouple the highly latent loads in outside air from the balance of the space conditioning system (See sidebar).
In large outside air fraction applications, like health care facilities, conventional systems handling the combined fresh and return airstreams can leave humidity loads unmet, especially at part-load conditions. Indoor humidity levels rise as a result and encourage microbial activity.
DOAS can neutralize outside air humidity loads, allowing conventional cooling technologies to handle the predominately sensible load downstream. DOAS technologies include cool and reheat, heat pipe or run-around heat exchange equipped cooling coils, dual path cooling coils, enthalpy exchangers, and desiccant dehumidifiers13.
Filtration
Generally, airborne pathogens increase in size from viruses (0.01 to 0.3 microns) to bacteria (0.2 to 2 microns) to fungi (1 to 20 microns)14. Physical size is the single-most important characteristic by which to assess filtration efficiency.Based on ASHRAE's new Minimum Efficiency Reporting Value (MERV) rating system for filters in Standard 52.2-1999, "Method of Testing General Ventilation Air-Cleaning Devices for Removal Efficiency by Particle Size," mechanical arrestance of fungi (spores) requires filters with MERVs of 9 to 12 (with up to 90% efficiency in the 3 to 10 micron range).
Mechanical arrestance of bacteria requires filters with MERVs of 13 to 16 (with up to 95% efficiency in the 0.3 to 1.0 micron range). Mechanical arrestance of high percentages of viruses requires high efficiency particulate arrestance (HEPA) filtration, beyond the range and outside the scope of the ASHRAE MERV rating tests.
The lower MERV-rated filters can be effectively applied as prefilters to reduce the larger particle loading on HEPA filters and extend the service life of the more expensive HEPA media. An essential aspect of proper filtration application is matching the health care space usage and contaminant control requirements with the proper filter selection 15.
Irradiation
The reemergence of TB as a national health issue has spurred a resurgence of UVGI applications in health care facilities. After early success with this technology in the 1920s and 1930s, the use of UVGI fell into disfavor after mixed results in the 1940s through 1950s due to poor designs or misapplications16.Though not presently sanctioned by health care construction and design guidelines, the "precursor" CDC infection control guidelines do recognize that "as a supplemental air-cleaning measure, UVGI is effective in reducing the transmission of airborne bacterial and viral infections in hospitals ... but it has only minimal inactivating effect on fungal spores"10 and bacterial spores16 .
In isolation rooms and some common areas like waiting rooms, UVGI is applied to upper air zones and its effectiveness is dependent on sufficient irradiation intensity, satisfactory air movement from the lower air zone, adequate airborne microbe exposure time, moderate room air humidity, and susceptibility of that microbe to UV. In AHUs, UVGI is applied in close proximity to the filter banks and the cooling coils. The UV emitters must have a line of sight to the targeted microbial amplification sites such as wet coils, drain pans, and moisture-exposed filters.
Finding the Right Combination
The optimal mix of these three technologies is not known. Life-cycle costs of HEPA filtration on recirculated air will tend to be more cost effective in hot, humid, or cold climates, while in mild or dry climates large volumes of outside air for ventilation can prove more economical. Combining ventilation air with filtration results in overall performance that is essentially additive and cost optimization becomes straightforward17, 18.
When all three technologies are applied together, UVGI can target vulnerable bacteria (excluding spores) and viruses in AHU microbial amplification sites and in ill patient containment areas; filtration can remove fungi (spores) from incoming outdoor air and provide the bulk of bacteria and virus removal in recirculated air; and ventilation can deliver dilution air from outdoors to further decrease concentrations of all airborne microbes promulgating indoors.
Conclusions
Many other very important aspects of health care facility IAQ engineering have not been addressed here due to the brief nature of this article. However, this overview has shown that fresh air ventilation (preferably with DOAS), filtration, and UVGI form the triad of technologies applied by engineers to provide an indoor environment that promotes both patient welfare and worker protection in health care facilities.These technologies allow multiple medical and building science practitioners to meet current and future guidelines for health care facility design, construction, and operation, including disease and infection control. Much work still lies ahead, though, to determine the preferred mix of technologies to optimize the overall economics while minimizing health risks associated with the resultant IAQ provided in health care facilities. ES
EDITOR'S NOTE: Some images associated with this article do not transfer to the Internet. To review the images, please refer to the print version of this issue.
"It's not the heat, it's the humidity!"
In every issue ofES, Michael Kjelgaard, P.E. gives us his Weather Report for two months ago. It is a constant reminder of the heating and humidification loads, cooling and dehumidification loads, and resultant energy costs, associated with processing outside air for ventilation.Back in 1997, the concept of the Ventilation Load Index (VLI)19 was introduced to provide a quick measure of the annual sensible and latent cooling loads in ton-hrs/cfm of outside air brought indoors to a space neutral condition (75 degrees F/50% rh). The VLI was a response to the increasing ventilation rates of ASHRAE Standard 62 and, in particular, the emerging problems with humidity control20 during the cooling season for buildings with large volumes of outside air, like health care facilities.
As shown in Figure 2 for three selected major cities in the United States, the separation of the sensible and latent components of outside air helped engineers "visualize the humidity control problem" by quantifying the individual magnitudes of the ventilation cooling loads associated with ambient temperature and moisture, while further fostering the introduction of DOAS technologies for ventilation air pretreatment as a solution.
At the same time as the VLI was introduced, ASHRAE was giving moisture loads present in outside air long overdue recognition in the 1997, and now 2001, edition of the ASHRAE Handbook of Fundamentals. The 1993 Handbook (Chapter 24) contained only cooling design drybulb temperatures (1%, 2.5%, and 5% summer conditions; replaced by 0.4%, 1%, and 2% annual conditions in 1997).
The 1997 edition (Chapter 26) introduced the design dewpoint temperature and design humidity ratio. This design humidity ratio, which occurs at lower drybulb temperatures, had been the long overlooked "other peak cooling condition." In fact, in nonarid climates, the cooling load resulting from outside air is larger at the design humidity ratio than at the design drybulb temperature as shown for Atlanta in Figure 3.
Works Cited
1. Nightingale, F.,Notes on Nursing, W. Parker & Son, London, 1859.2. Nightingale, F.,Notes on Hospitals, W. Parker & Son, London, 1859.
3. Streifel, A. J., “Health-Care IAQ: Guidance for Infection Control”,HPAC Engineering,October 2000: 28-36.
4. Spengler, and McCarthy, “Indoor Environmental Quality in Hospitals,”Indoor Air Quality Handbook,McGraw-Hill, New York, 2001.
5. American Institute of Architects (AIA), “Hospitals: Mechanical Standards,”Guidelines for Design and Construction of Hospital and Health Care Facilities, Washington, DC, 2001.
6. American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), ANSI/ASHRAE Standard 62, “Ventilation for Acceptable Indoor Air Quality,” ASHRAE, Atlanta, 2001.
7. American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), “Health Care Facilities,”Applications Handbook,ASHRAE, Atlanta. 1999.
8. Centers for Disease Control (CDC), “Air,” Draft Guideline for Environmental Infection Control in Health care Facilities, CDC, Atlanta, 2001.
9. ASHRAE, “IAQ Seminar,” seminar notebook, 1986.
10. Noskin, G. A. and L. R. Peterson, “Engineering Infection Control through Facility Design,”Emerging Infectious Diseases, March-April 200: 354-357.
11. Cali, S., J. Franke, L. Conroy, and P. Scheff, “First, Do No Harm: Indoor Environmental Quality Air Quality (IEQ) in Hospitals,”EM-Environmental Management, October 2000: 22-29.
12. Ninomura, P. and J. Bartley, “New Ventilation Guidelines for Health-Care Facilities,”ASHRAE Journal, June 2001: 29-33.
13. Gatley, D. P., Dehumidification Enhancements for 100-Percent-Outside-Air AHUs Parts 1 – 3,”HPAC Engineering,September: 27-32, October: 51-59, November: 31-35.
14. Bahnfleth, W. and W. J. Kowalski, “Airborne Respiratory Diseases and Mechanical Systems for Control of Microbes,”HPAC Engineering, July 1998: 34-48.
15. Burroughs, H. E., “The Art and Science of Air Filtration Management in Health Care,”HPAC Engineering, August 1998: 79-86.
16. Bahnfleth, W. and W. J. Kowalski, “UVGI Design Basics for Air and Surface Disinfection,”HPAC Engineering,January 2000: 100-110.
17. Dragan, A., “HVAC Design Approach and Design Criteria for Health Care Facilities,”ASHRAE Transactions, MN-00-8-2, 2000.
18. Dragan, A., “Comparative Analysis of HVAC Systems That Minimize the Risk of Airborne Infectious Disease Transmission,”ASHRAE Transactions, MN-00-8-4, 2000.
19. Harriman III, Lewis G. and D. R. Kosar, “Dehumidification and Cooling Loads from Ventilation Air,”ASHRAE Journal, November 1997: 37-45.
20. Kosar, D. R., M. J. Witte, and D. B. Shirey, “Dehumidification Issues of Standard 62-1989 [ANSI/ASHRAE Standard 62-1989, Ventilation for Acceptable Indoor Air Quality],” ASHRAE Journal, March 1998: 71-75.

